Local
41 HIV, PrEP patients sue Gilead
They claim pharmaceutical company sought profits over patient health


(Gilead logo)
Pharmaceutical giant Gilead was hit with a lawsuit filed by 41 people from 12 states on April 11 who allege they suffered bone and/or kidney damage after taking Gilead’s tenofovir disoproxil fumarate, (TDF). The personal injury suit, filed in California Superior Court in Los Angeles, alleges the pharmaceutical company knew in 2001 that TDF was “highly toxic in the doses prescribed and risked permanent and possibly fatal damage to the kidneys and bones” —and that there was a safer alternative.
“More and more plaintiffs are coming forward to tell their stories of how they have been harmed by Gilead’s practice of putting profits over patient health,” said Liza Brereton with HIV Litigation Attorneys.
The suit is part of an ongoing effort to hold Gilead accountable for its alleged failure to rectify a known defect in the drug formulation of TDF, knowing that a safer alternate existed—tenofovir alafenamide (TAF). Additionally, AIDS Healthcare Foundation spokesperson Ged Kenslea told the Los Angeles Blade, the suit also includes Gilead’s failure to warn patients of TDF’s damaging side effects and its active misrepresentation of TDF’s efficacy and substantial risks. AHF is paying for the litigation and only seeks legal costs in return.
“Gilead had their safer alternative available and suppressed it in a malicious deliberate way for over 15 years just so they could maximize and extend the profits on TDF,” Kenslea said.
The suit also asserts that Gilead deliberately and maliciously kept information about TAF quiet to extend its patent, FDA exclusivity, and sales of its existing medications, including TDF. Gilead netted over $18 billion in profit in 2015.
The lawsuit alleges that “thousands and thousands of HIV/AIDS patients may have been unwittingly exposed to significant kidney and bone damage from TDF” during their antiretroviral drug regimens. Additionally, “many HIV-negative individuals seeking to prevent HIV acquisition may have suffered similar harm to their kidneys or bones from taking Truvada as part of their PrEP (pre-exposure prophylaxis) protocol.”
Gilead sells TDF under the brand name Viread. TDF is also a component of Gilead’s Truvada.
“Gilead knowingly continued selling a drug that had debilitating side effects while keeping a safer version in the wings until the patent on the first, more dangerous version had been exhausted. In other words, Gilead allowed serious injuries to occur so they could make a few more bucks before releasing a safer version,” prominent AIDS activist and “My Fabulous Disease” blogger Mark S. King told the Los Angeles Blade. “Meanwhile, Gilead continues to own our governmental and community-based HIV response lock, stock, and barrel by throwing endless cash at national organizations that should have our best interests at heart. Gilead’s behavior has been truly vile.”
King added: “I have been critical of AHF in the past for the quickness to sue, primarily other HIV organizations with which they differed. But this lawsuit is righteous and I hope they win, big time.”
a&e features
A king rises in Vico Ortiz’s new solo show
With a little bit of ‘astrology woo-woo-ness, a little bit of magic woo-woo-ness, drag and fabulosity,’ they tether together the story of the relationship between them and their mother

Nonbinary, Puerto Rican icon, comedian, actor and activist, Vico Ortiz, 33, binds and weaves awkward childhood moments, family expectations, Walter Mercado and the love of their life embodied by a household mop, to tell the story of the rise of a king.
During the peak of Pride month, Ortiz gifted the Los Angeles queer and trans communities with a spectacularly queer, solo show featuring themself in their quest of self-discovery, a profound sense of connection and reconnection with their femininity and masculinity through growing pains and moments of doubt. This is a show that Ortiz describes as “wholesome, but burlesque.”
King Vico Ortiz rises
The show, which premiered on June 12, details Ortiz’s childhood, vignetting and transforming through their most formative years and through canon events that led them to their gay awakening, such as watching Disney’s Mulan (in Spanish) and the moment Ortiz cut their hair in honor of the scene where Mulan cuts hers off. Ortiz took the audience on a journey through their inner monologue during the moments in their childhood and into adulthood, where they not only come to terms with their identity, but also learn to understand the internal battle their parents went through as they watched their king rise.
Though Ortiz mostly only acted prior to writing and producing their first solo show, they finally took their opportunity to do things a little differently. Last June, their friend Nikki Levy, who runs a show called Don’t Tell My Mother, coached Ortiz to dredge up childhood memories and tether them in a way that could be told and understood by an audience as a show about queerness and self-discovery.
“[Levy] started asking me those questions that dig deeper into the emotional journey of the story, not just ‘hehe’ ‘haha’ moments, but there’s something a little deeper happening,” said Ortiz in an interview with the L.A. Blade. This is when they asked Levy to help coach them through the process of putting the story together in a way that Ortiz imagined it, but also in a way that made sense to the audience.
Ortiz says that with a little bit of ‘astrology woo-woo-ness, a little bit of magic woo-woo-ness, drag and fabulosity,’ they tether together the story of the relationship between them and their mother.
“Astrology had a huge influence in my life growing up from the get-go,” said Ortiz. “I was born and [my mother] printed my birth chart.” The Libra sun, Sagittarius rising, Scorpio moon and Venus in Virgo, says they have always known their chart and that not only did astrology play a huge role in their life growing up, but so did astrologer-to-the-stars Walter Mercado.
The solo was partly influenced by their mother and partly influenced by the iconic, queer, androgynously-elegant Mercado, who famously appeared on TV screens across homes in Latin America and the United States for a segment on that day’s astrological reading.
Seeing Mercado on that daily segment shaped Ortiz’s view of gender and began to understand themselves in a new-found light — one in which they saw their most authentic self.
“Seeing that this person is loved and worshipped by all these people who are like: ‘we don’t care that Walter looks like Walter, we just love Walter,’” said Ortiz. This is when they realized that they too, wanted to be loved and adored by the masses, all while fully embracing their masculinity and femininity.
Closing Night of ‘Rise of a King’
The closing night show of ‘Rise of a King’ was a reminder of how unpredictable life can be and how darkness comes in on some of our brightest moments. Ortiz brilliantly pulled off an improv monologue during a 15-minute power outage. Though it was unpredictable, it was on theme. Ortiz owned the stage, going on about childhood memories that shaped them into who they are today and how they have reconnected with the imaginative child that once told the story of a half-butterfly, half-fish.
The rest of the show went according to plan, immersing the audience in a show that took us straight into the closet of Ortiz’s parents and where Ortiz not only discovered, but learned to embrace who they truly are — to the first moments they embraced the king within and outwardly began to show it to audiences, and eventually their mother.
The set, designed by Jose Matias, functioned as a walk-in closet that transforms throughout the show against the backdrop of drawings of Ortiz and their family memories projected on a big screen on the stage.
Ortiz’s original, solo-show had its world premiere at FUERZAfest in New York City, then its west coast premiere at L.A.’s own Hollywood Fringe Festival.
Follow @puertoricanninja for more updates on their upcoming work.
California
Williams Institute reports impact of deportations on LGBTQ immigrants
Latest report suggests transgender, nonbinary and intersex immigrants face significantly higher safety risks
Williams Institute at UCLA has released its latest report, highlighting the intersection between LGBTQ and immigration issues and the impact of the U.S. Customs and Immigration Enforcement (ICE) raids across Los Angeles on LGBTQ people.
According to the brief, LGBTQ immigrants who hold legal status, but who are not naturalized citizens may also face challenges to their legal right to reside in the U.S.
Recent reports indicate that non-citizens with legal status are being swept up in immigration operations and several forms of legal status which were granted at the end of the Biden administration are being revoked. Those include: Temporary Protected Status (TPS) for some Venezuelan immigrants, as well as those from Afghanistan and Cameroon, while Haitian nationals are now facing shortened protection periods, by up to six months.
The Justice Department has proposed a new rule which grants the government border authority to revoke green card holders’ permanent residency status at any time. This rule is currently under review by the Third Circuit Court of Appeals, which could significantly affect non-citizens who are currently documented to reside in the county legally.
Supervisorial District 1, under Supervisor Hilda L. Solis, and Supervisorial District 2, under Supervisor Holly J. Mitchell would particularly be affected as it contains the city center of Los Angeles and nearly 29,000 LGBTQ, noncitizens would face the harshest impact. Those two districts contain many of the county’s historically Black, Latin American and Asian, Pacific Islander neighborhoods.
For transgender, nonbinary and intersex immigrants arrested or detained by ICE, there are additional impacts regarding how federal law defines biological sex and gender identity. The Trump administration has signed an executive order which redefines “sex” under federal law to exclude TGI individuals. This adds an extra thick layer of possible violence when TGI individuals are placed in detention centers or in holding that does not correspond to their identity.
According to the report, ‘transgender, non-binary, and intersex immigrants must navigate an
immigration and asylum system without information about how federal agents will respond to their gender identity and with the risk of greater violence if placed in detention centers, given the effects of this executive order.’
The brief estimates the number or foreign-born adults in Los Angeles County who will be potentially affected by the Trump administration’s executive orders on mass deportations.
Graphic courtesy of Williams Institute at UCLA.
Using previous data from other Williams Institute Studies and reports from the University of Southern California Dornsife Equity Research Institute and data from the Pew Research Center, the latest brief states that there are over 1.35 million LGBTQ-identifying people across the U.S., with 30% of them residing in California.
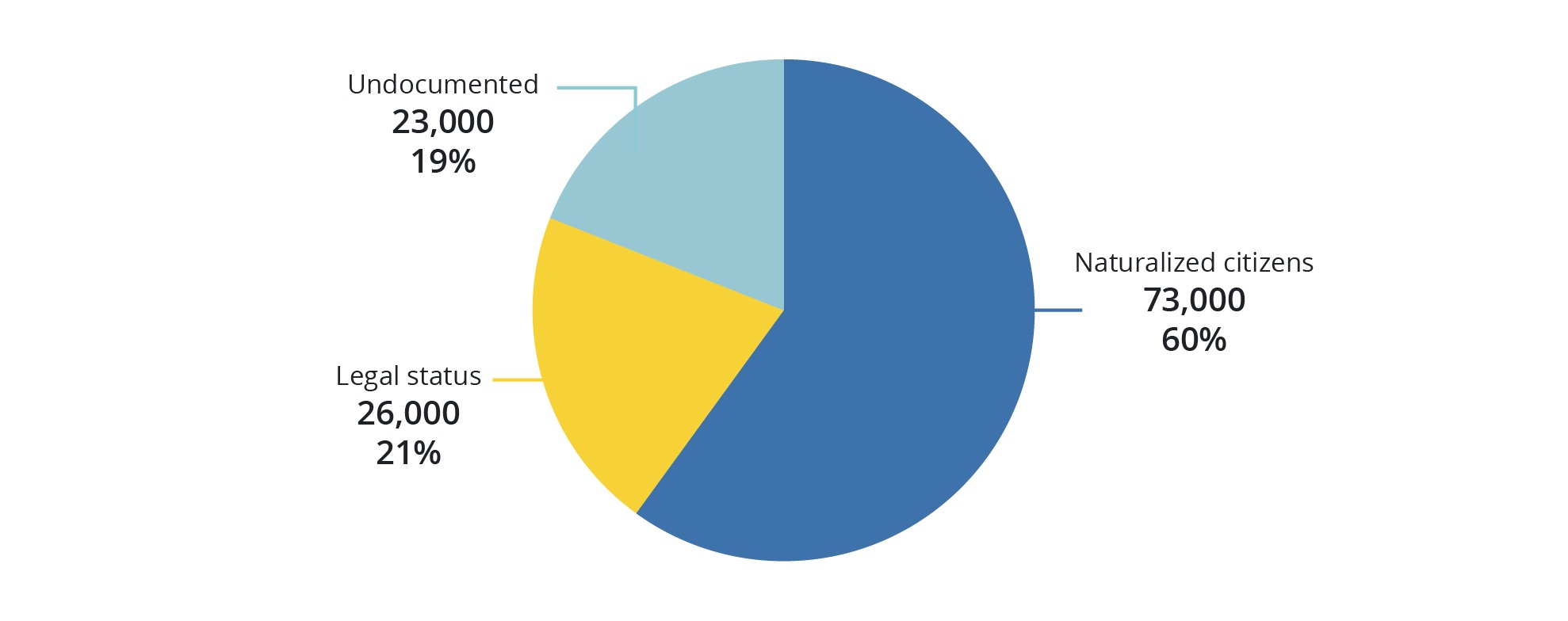
The report further points to 122,000 LGBTQ immigrants who reside within LA County specifically, making Los Angeles County home to about 10% of all LGBTQ adult immigrants in the U.S.
While 18% of those Angelenos are foreign-born, only around 7%, or 49,000 of them do not hold legal status.
Using research from the Pew Center and applying an estimate, that means that there are approximately 23,000 undocumented LGBTQ across LA County and the remaining 26,000 LGBTQ immigrants in the county have some form of legal status.
Among the LGBTQ population of adult immigrants in California, approximately 41,000 are transgender or nonbinary. That figure also points toward approximately 5,200 of them residing in LA County. According to the proportions applied for this estimate, the Williams Institute approximates that around 3,100 transgender and nonbinary immigrants in LA County are naturalized citizens, over 1,100 have legal status and just under 1,000 are undocumented.
According to a brief released in February by the Williams Institute, ‘mass deportations could impact 288,000 LGBTQ undocumented immigrants across the U.S.
California
Trinity Park, a foundation of memories from childhood to present day
‘The park, in a sense, saw me grow up and helped me build a close sense of community at a time when I felt very alone and isolated’

In the heart of South Central Los Angeles lies Trinity Recreation Center, known as Trinity Park, considered what some might refer to as a “hood staple.”
Although there is no denying the park’s history of gang-related incidents and even death, for many, the park is also a place full of profound memories, cherished as one of the only green spaces and gathering places within a community that is often underserved. The park sits in the city’s 9th Council District (CD-9), a district known for its lack of green spaces.
South Central L.A. is often characterized by an over concentration of liquor stores and a lack of quality, affordable grocery stores and sit-down restaurants, contributing to high rates of heart disease, diabetes and premature death among South L.A. residents.
For me, the park brings me back to a time when life was easier. It was the place where I made my first friend. This park was my large family’s front yard for over three years and a place that I have passed by and walked through for over two decades.
Trinity Park was one of the first places I have memories of. A year after my family immigrated to the U.S., we moved into a three-bedroom house across the street from the park. The first year in the states was difficult for my mother, who juggled two jobs.
For me, understanding very little English and not being able to speak it made it extremely difficult to communicate or connect with other kids and adults at school. My thick accent was always a point of concern and embarrassment.
Trinity Park, which in the early 2000s was even more notorious for crime and shootings, was a special place for my family and me during the almost three years that we lived neighboring it.
When my mother worked long hours, my aunts and uncles would take me to the park at least twice a week, where I would run uncontrollably and ride the swings for as long as possible. The park, in a sense, saw me grow up and helped me build a close sense of community at a time when I felt very alone and isolated.
That, too, is what the park has been to many of the kids and community living in the park’s surroundings. Trinity Park, is also located 12 minutes from downtown L.A., in an area of South Central L.A. known as Historic South Central within CD-9, where the majority of the population is composed of Black and Latino working-class families.
The Trinity Recreation Center opened its doors in 1968. In the last few years, there have been significant upgrades and additions to the park, including the 2016 opening of a synthetic soccer field and a skate park, which officially opened in 2021.
To what to many outsiders might look like an unsafe and unpredictable piece of land, Trinity Park holds dear memories for many of those who have passed through or hung out in the park at some point in their lives.
The park was also where I remember making my first true friend in L.A., a friendship that wasn’t sparked because we went to the same class or because our parents knew each other, but because of a simple connection between kids while playing. I don’t remember her name, but the photo below was taken the day I met her at Trinity Park.
My mother, on one of her only days off, had taken me to the park. It was the summer of 2004 and we arrived at the park close to sunset. I wish I could recall more than us playing tag and running around the park, but I remember the feeling of making my first friend, one I had made on my own. We probably spent three to four hours together that day playing and at the end of it all, my mother ran across the street to bring her camera. I think she, too, wanted to preserve the memory, the feeling of seeing her daughter connect with another kid after what had been a very tough time in both of our lives.

Brenda’s friend she made in Trini Park (L) and Brenda (R) pose together at Trinity Park in 2004.
(Photo courtesy of Brenda Fernanda Verano)
That was also the park where I taught myself to ride a bike and that gave me confidence, something that was hard for me to find as a kid.
Later in life, although we moved out of the home across the street from the park, my family and I continued living in that same neighborhood and all throughout my high school journey, I walked through that park every day to get to school. In middle school, the park was also a meetup place for my friends and me after school, where we would hang out and share music playlists.
The park has been a silent but consistent entity from childhood to now — adulthood — always in the background of pieces and times in my life.
EDITORS NOTE: This article was published through the Bezos Fellowship grant provided by the Ethnic Media Services, which recently changed its name to American Community Media. The article was written by Brenda Fernanda Verano, an award-winning journalist and reporter for CALÓ News, a local non-profit newsroom focusing on the Latin American community of Los Angeles.
Local
Trans Lifeline named best nonprofit by LA Blade readers: A lifeline of love in a time of crisis
‘Being voted Best Nonprofit of the year carries heavy symbolic weight’
Trans Lifeline has been named Best Nonprofit by readers of the LA Blade, a powerful nod to the trans-led organization’s work supporting the community through some of the most challenging years in recent memory.
The organization, which operates as a peer-run crisis hotline and resource network for trans people, was founded on the belief that no one understands what trans people need better than trans people themselves. Now North America’s largest trans-led direct service provider, Trans Lifeline offers peer support, microgrants, and advocacy for trans individuals in crisis, with every service shaped by lived experience and radical care.
In June 2024, Trans Lifeline welcomed kai alviar horton* as Executive Director. horton’s leadership has been defined by a deep commitment to love, liberation, and community care. Under his guidance, the organization has prioritized internal health, invested in hotline and support teams and fostered a culture where trans leadership, particularly from Black, Indigenous, and other communities of color, thrives.
The past year brought growth, but also unimaginable grief. In 2024, over 533 anti-trans bills were introduced at the state level and more than 80 at the federal level. Trans Lifeline stood firm in its mission amid the onslaught of legislative attacks. At the same time, the community mourned the loss of at least 350 trans people, many of them Black and brown trans women and femmes. For the team at Trans Lifeline, those losses are not just statistics. They are personal, collective and deeply felt.
“We could never have imagined the depth of connection, healing, and resilience we would experience together,” said Myles Markham, speaking on behalf of the organization. “There are no words to capture that grief. Through it all, Trans Lifeline has continued to serve as a symbol of unwavering love.”
That love is translating into urgent, expansive action. Early into 2025, Trans Lifeline experienced a 400% increase in call volume. To meet the moment, the organization scaled up operations by hiring more operators, launching a new text-based support line and training a fresh cohort of volunteers. They’ve also begun rebuilding the infrastructure for their beloved microgrants program, with plans to relaunch it before the end of the year. The program provides direct financial assistance to trans individuals, part of the group’s core commitment to economic justice.
Being voted Best Nonprofit of the year carries heavy symbolic weight. “This award gives Trans Lifeline prominent exposure among Angelinos who are LGBTQ, allied, or seeking support,” said Markham. “It signals to our donors, partners, volunteers, and crisis service users that the organization is valued and trusted by the trans community.”
Recognition like this is especially meaningful for peer-led organizations.
“It goes such a long way in demonstrating that investing in work led by those directly impacted is the only way forward,” they added. “The Blade’s award reinforces the value of our lived experience and our service to trans people in crisis.”
And for trans youth, their message is simple and unwavering: “It’s okay to not feel okay right now. We are in your corner today and forever. You can call or text us at (877) 565-8860—whether you’re in crisis or not, even if you’re not sure if you’re trans. We love you and are here to support you through it all.”
To support TransLifeline visit their website.
Breaking News
ICE raids cause civil unrest in Los Angeles during Pride month
Thousands of National Guard members and Marines are now being deployed to Los Angeles with intention to occupy for the next 60 days

ICE raids have taken place across Los Angeles County over the last few days and tens of thousands of Angelenos have taken to the streets to protest against the raids and the police brutality involved in the arrests.
The Trump administration has threatened to arrest Governor Gavin Newsom and L.A. Mayor Karen Bass if they were to interfere with the ICE raids. In response, California has now filed a lawsuit against the Trump administration.
Early Monday morning, the U.S. Northern Command announced that it activated around 700 Marines, after the Pentagon and the Trump administration deployed around 2,000 National Guard troops to Los Angeles over the weekend. As of today, Trump has deployed double the amount of National Guard troops and ICE raids are said to continue for the next 30 days. The deployment is set to cost $134 million and last 60 days or more according to Secretary Pete Hegseth and a senior defense official.
Reporters have been hit with rubber bullets, batons and have been tear gassed while trying to document the protests. There are eyewitness reports and video footage showing police officers trampling people over with horses, running people over with squad cars and detaining people who have legal status.
In a broadcast interview with CNN, Mayor Bass stated that she believes that if ICE raids hadn’t happened on Friday, we would not be seeing the type of disorder we are seeing. The Los Angeles Police Department declared Downtown L.A. an unlawful assembly area after union president David Huerta was detained by ICE along with several undocumented immigrants. Huerta appeared in court on Monday and was released on a $50,000 bond.
LAPD Chief Jim Donnell says they have adapted their tactics to arrest people, but that they are ultimately “overwhelmed” by the number of protesters.
“We have adapted our tactics to take these people into custody and to be able to hold them accountable,” said Chief Donnell. “We are overwhelmed as far as the number of people out there engaged in this type of activity,”
Mayor Bass said she was “completely in sync” with what the police chief stated, adding that she believes there is enough video footage to prosecute protestors even if they did not get arrested on scene.
“Some people might think that just because they haven’t gotten arrested on the spot, that they’ve gotten away with it and the message I would send is: there’s ton of video tape and people who didn’t get arrested today for committing violent acts — don’t plan on the fact that you get off because you can get arrested in the next few days,” said Mayor Bass.
Mayor Bass doubled-down on her statement regarding the ICE raids and how L.A. is a city of immigrants and ICE raids will continue to affect the local economy.
There have also been reports that ICE raids are taking place across schools and graduation ceremonies.
Los Angeles Unified School District is set to deploy school police to set up safe zones around graduations and school campuses amid these raids targeting celebrations. According to the LA Times, school police will patrol and guard campus entrances when ICE and Border Patrol are seen in the area. Graduation ceremonies will become sanctuaries for families until immigration agents disperse from the area.
Medical providers like St. John’s Community Health released a statement on the issue.
“The aggressive increase in ICE activity is forcing already vulnerable people to fear going to the doctor, school, or even the grocery store — and putting countless families in danger,” said Jim Mangia, president and CEO of St. John’s Community Health.
Community leaders like Tony Hoang, executive director at Equality California stated that as a child of immigrants, it deeply saddens him to see the ICE raids take place across Los Angeles.
“Equality California joins Governor Newsom and Attorney General Bonta in calling for an end to National Guard deployment. We condemn the raids that have occurred and are continuing, which are xenophobic and traumatizing to families, individuals, and communities,” he said.
“We stand in solidarity with immigrant communities across Los Angeles and the state—and we call on every leader, at every level of government, to reject this assault on our values and take urgent action to protect those under threat.”
Los Angeles
LA Black Pride: ‘We are no longer waiting to be seen’
Joy as power, presence as protest, visibility that refuses to be diminished

As Los Angeles Black Pride (LABP) gears up for another saucy season of celebration, culture, and resistance, we are proud to announce a new six-week media partnership between LABP and the Los Angeles Blade. This collaboration is not just promotion but intention. It is about making sure Black queer voices are not just heard, but honored and amplified.
To kick it off, we are excited to announce that Saucy Santana will headline LABP’s Saturday night main stage. Known for their unapologetic energy, queer-centric bops, and fearless showmanship, Santana represents exactly what LABP is about — joy as power, presence as protest, and visibility that refuses to be diminished.
The theme for LABP 2025 is “Black Queer Futures Are Now: We Are No Longer Waiting to Be Seen.” Our partnership sets the tone for what’s to come. It is a shared commitment to telling Black queer stories, past and present, and investing in what’s to come.
What started as a party born out of necessity has now become a full-scale movement. LABP Executive Director Brandon Anthony, who began his journey throwing parties like Ice Cream Thursdays, recounts the roots of this project:
“It started off as something I felt was missing… a space that felt like us: where the music hit right, where the energy felt familiar, and where we could just be,” he says. “What began as a vibe we needed grew into a platform. Now, it’s a business, a brand, a movement, but at the heart of it, I’m still just someone who wanted to create space for my community to feel good, feel seen, and feel proud.”
From nightlife to nationwide recognition, LABP is proof that when Black queer folks create for themselves, the result is not just representation, it’s revolution. In a landscape where many Pride events still sideline Black and Brown voices, LABP has become a necessary act of reclamation.
“Because if we don’t, who will?” Anthony asks. “Too often we get left out or placed in the background. Our energy, our style, our voices — we drive the culture. When we center ourselves, it gives others permission to do the same. Joy is more than a feeling, it’s a form of resistance.”
That resistance has never been more needed. From limited funding to systemic erasure, LABP continues to thrive against the odds. But the message is clear: thriving should never necessitate struggle.
“We’re not just asking for visibility — we’re asking for the tools to thrive,” he explains. “Now more than ever, we need partners who are aligned with the people, not just the optics.”
With the 2025 theme of “Legacy and Leadership in Action,” LABP honors the trailblazers who paved the way. Icons like Jewel Thais-Williams, founder of the legendary Catch One, are celebrated annually through the Jewel Thais-Williams Award.
“Catch One wasn’t just a nightclub, it was a safe haven,” Anthony shares. “Legacy isn’t just about the past. It’s about lifting up the folks doing the work right now and keeping that energy alive.”
LABP continues that work through programming that extends far beyond June. Year-round initiatives include pop-up markets, health services, creative workshops, and political advocacy.
“One of the moments that really showed what we stand for was the All Black Lives Matter march in 2020,” he says. “We co-led it alongside Gerald Garth, and it was powerful to see thousands show up for Black Trans lives. That wasn’t just a moment – it was a movement.”
Whether it’s showcasing emerging artists on stage, uplifting Black trans creatives, or building platforms for new leaders, LABP is focused on making sure the next generation has room to grow.
“When people are given a platform to show what they can do, it creates more than visibility, it creates momentum,” he says. “That’s what keeps everything moving forward.”
And with names like Saucy Santana taking center stage, that movement is gaining speed. Santana’s headlining performance isn’t just a concert — it’s a declaration. It says that Black queer talent is main-stage worthy, every time. This partnership is not performative – it’s purposeful. It’s a bridge between platforms, audiences, and shared values.
“LA Blade has a huge reach, and by choosing to amplify Black queer voices, they’re helping bridge gaps and build deeper understanding,” says Anthony. “This isn’t about charity or tokenism. It’s about showing the world who we are, what we’re building, and why it deserves to be seen.”
In the words of LABP’s ongoing mission: We are no longer waiting to be seen. We are building what’s next.
Local
WeHo Council member Erickson launches bid for California Senate seat
Says he wants to fight for LGBTQ rights, the environment, and lower cost of living

Citing the need for experienced leadership to protect LGBTQ rights and control the skyrocketing cost of living in Los Angeles, West Hollywood City Council member John Erickson has announced a bid for the California Senate District 24 seat that will be up for election in November 2026.
“I’m running for California state Senate because after what I’ve seen, not only from the first few months of the Trump administration, the devastating fires, but also the impact of what we’re seeing on our state budget from federal and state budget cuts,” Erickson says.
“And as an LGBTQ elected official, and someone who’s lived paycheck to paycheck and deals with housing insecurity because I live in a rent-controlled apartment, I understand the different ways that the rising costs of living impact our lives. We need someone up there who lives these realities.”
The sprawling district, which includes West Hollywood, Hollywood, Malibu, Agoura Hills, Santa Monica, and many of the South Bay cities, is currently represented by term-limited Sen. Ben Allen. Erickson says his experience on WeHo City Council makes him an ideal representative for this diverse collection of communities.
“That’s why West Hollywood is such a great test case for it, because we have so many different communities and populations,” he says. “All of these different locations, they all make up people who are really well versed in what’s going on and progressive, but also wanting to make sure government’s working for them.”
While California has long been a leader on LGBTQ rights, Erickson says it’s still important to have queer representatives at the state level.
“Having more representation is always critical, right? If you see it you can believe it and you can achieve it,” he says, citing his proven leadership on West Hollywood City Council.
Erickson says the attacks on LGBTQ rights are intensifying both from the federal government and the courts, and California needs lawmakers who are prepared to stand up for us.
“There are challenges in the state of Texas that are trying to make PrEP and PEP completely illegal to get, so we need to make sure that we’re doing all that we can to make sure access to that is available,” he says.
And when it comes to trans rights, Erickson is unequivocal in his support for trans people, despite growing hostility from the federal government, and Gov. Newsom’s recent calls for trans women to be excluded from sports.
“We can never and should never put equality on the chopping block for any member of any community,” he says. “We have seen individuals playing in sports who have full rights and dignity of who they are in their lives and that needs to be honored and uplifted.”
Erickson says he has a plan to stand up for trans inclusion and equality at the state and federal levels.
“I would advocate for the full inclusion of budgetary dollars as well as fighting for their rights either through legislation or supporting litigation that the state would be engaged in,” he says.
Of course, the District also faces huge challenges related to the skyrocketing cost of living and rebuilding from this year’s devastating fires, which Erickson says are related problems: The fires have exacerbated a housing shortage while driving up the cost of insurance for everyone in the region.
He says he wants to ensure that state and federal resources are directed at clearing fuel from fire-prone areas to prevent more fires
“We need to make sure that our partners in the federal government also aren’t removing any funds from us. The federal advocacy that the states can be playing to ensure that California is getting not only our rates from FEMA but the monies that we’re owed is critical,” he says.
On insurance, Erickson says the state has to focus on reducing costs for businesses while also investigating a state-run insurance program.
“California regulates insurance, and so, we can create a California-based insurance program that I know there’s been some talk about,” Erickson says. “California is the fourth largest economy in the world. We have the power to throw our weight around in that way.”
Erickson has come out as a supporter of the so-called “Abundance Agenda” that advocated for removing government obstacles from the producing things that people want more of – whether that’s housing or public works projects like transit and infrastructure – as way of bringing down costs and stemming the tide of people leaving California.
“The abundance agenda has allowed us to say we’re in a housing shortage,” he says. “Very few people can live in the communities in which they work. That’s unacceptable. Communities need to be investing in public infrastructure, streets, trees, and sidewalks, and investing in the expansion of metro or bus.
“We’re in a shortage, I think, of common-sense policy reforms”
Erickson has long been a supporter of West Hollywood’s dream for a Metro K-Line extension through the city, which he says will help reduce traffic and improve mobility while reducing people’s day-to-day costs.
“I think as more and more communities are getting activated and frustrated around the lack of, Moment on these issues. I’ve seen the success of the movement.”
He also cites the current slump in the local film and TV industries as evidence that the state needs to reduce the cost of doing business.
“They’re leaving California over all of these costs of doing business skyrocketing,” he says.
Erickson is joining a crowded field seeking the Senate seat. Already having declared their candidacy are Palos Verdes school board member Eric Alegria; Doheny-Sunset Plaza neighborhood council president Ellen Evans; journalist Brian Goldsmith; LA Human Relations Commission member Brittany McKinley; LA Planning Commission member Mike Newhouse; and cardiologist Sion Roy, all Democrats. The sole declared Republican candidate is Palisades Charter High School trustee Kristina Irwin.
Local
Andrew Bear on Pride Night Out and the power of resistance
Silver Lake event offers intimate vibes rooted in community

In a city loaded with Pride celebrations, Pride Night Out has carved out a space all its own. The vibes are intimate, intentional, and solidly rooted in community. What began as a modest backyard get-together in the dreamy neighborhood of Silver Lake has since bloomed into one of LA’s most relevant queer events bringing together trailblazers, tastemakers, and allies for an evening of panel discussions and more. It effortlessly blends art, connection, and resistance with its own signature style.
At the wheel of this Pride-centric powwow is Andrew Bear, a creative force behind the event and co-founder of Hyperion LA, a queer-inclusive production company that has made itself known for its storytelling and cultural impact.
In our conversation, Bear shares the origins and evolution of Pride Night Out, the philosophy behind this year’s theme “Why Now?”, and how the event blends queer history, art, and joy in a time of political urgency. From honoring legacy folks like the Queen Mother of the Imperial Court to creating intentional moments of celebration, Bear shares what it means to authentically lead with care and courage in today’s queer cultural landscape. For more information about the event, visit hyperionla.com/pride-night-out.
LOS ANGELES BLADE: First things first – can you tell us a little bit about the origins of Pride Night Out? What inspired the very first event?
ANDREW BEAR: Pride Night Out started as a passion project. I wanted to create a space that felt curated, intentional, and warm — a place where queer creatives, founders, and culture-drivers could gather outside of the usual nightlife or corporate mixers. It began with a few friends and collaborators in a Silver Lake backyard and has since grown into something that feels meaningful, magnetic, and still deeply personal.
BLADE: Pride is celebrated all across Los Angeles during Pride month. What sets Pride Night Out apart?
BEAR: Pride Night Out is about depth, not scale. We keep it focused so the energy stays high and the connections feel real. It’s not a party just for the sake of it. It’s an intersection of creative minds, queer visionaries, and people who actually want to build community. It’s stylish, celebratory, and intentional and being hosted in our own space makes it feel rooted in something bigger than a single night.
BLADE: What does the theme of this year’s panel, “Why Now?”, mean to you both personally and communally?
BEAR: “Why Now?” is a question I’ve been asking myself all year. For me, it’s about no longer waiting — to speak, to act, to lead. As a community, it feels like we’re being called to respond to the moment we’re in — politically, culturally, creatively. The theme is an invitation to be bold and honest about what matters most right now, and why we can’t afford to wait.
BLADE: The Queen Mother of the Imperial Court is scheduled to make an appearance. How are you incorporating queer legacy and lineage into this otherwise forward-looking event?
BEAR: Queer history lives in the room, whether we name it or not but we wanted to name it. Having the Queen Mother join us is such an honor. This year, we’re intentionally blending legacy and next-gen voices, not just in the programming, but in the energy of the night. It’s about remembering who came before us while making space for what’s next.
BLADE: How does Hyperion LA’s identity as a queer-inclusive production company inform the way Pride Night Out is produced?
BEAR: It’s not performative — it’s in our bones. From the way we staff the event to the brands we partner with, Pride Night Out is produced through a lens of inclusion, storytelling, and beauty. At Hyperion, we’re not just producing content, we’re producing culture. That same care and intention shows up in every detail of the event.
BLADE: How does art, performance, and nightlife affect or impact queer resistance today? Is this part of the intention behind the music + vibes portion of the evening?
BEAR: Nightlife has always been a site of resistance for queer people. Music, performance, and fashion have long been our weapons and our balm. The vibes are absolutely intentional — they create space for release, for joy, for connection. That’s powerful. That’s political. That’s Pride.
BLADE: The queer scene in Silver Lake has a rich and unique history. What makes the Silver Lake queer community feel different from other parts of LA?
BEAR: There’s a scrappy beauty to Silver Lake. It’s layered — historic, creative, a little chaotic, but always evolving. The queer community here feels deeply invested in each other. It’s not just where we live, it’s where we organize, collaborate, and care. It’s one of the last neighborhoods in LA that still feels like a neighborhood.
BLADE: What role do media and visibility play in moments like this? What are your hopes for the coverage/amplification of the event?
BEAR: Visibility is everything, especially now. Media coverage isn’t just PR, it’s preservation. It says we were here, we gathered, and we created something worth documenting. I hope the coverage shows the depth of the night — the fashion, the feeling, the power of queer people showing up for each other.
BLADE: In a climate where anti-LGBTQ+ legislation is spreading like wildfire, how do you balance celebration with cultural urgency?
BEAR: By refusing to separate them. Celebration is resistance. Our joy is not apolitical — it’s necessary. Pride Night Out isn’t escapism, it’s energy. We honor the urgency of this moment by creating space for people to recharge, reconnect, and reimagine together.
BLADE: What’s one thing you hope guests walk away with at the end of the night?
BEAR: Clarity. Whether it’s clarity around their purpose, their people, or just the reminder that they’re not alone. And maybe a little glow, from the lighting, the conversation, the Champagne, all of it.
BLADE: And last but not least, how has producing Pride Night Out changed or reaffirmed your own connection to queer identity and leadership?
BEAR: It’s made me braver. It’s shown me that queer leadership doesn’t have to follow a blueprint – it can be stylish, tender, disruptive, and unapologetic. This event keeps me connected to the why behind everything I do. It’s both a mirror and a love letter.
California
New California trans athlete policy creating ‘co-winners’ is a crock
You didn’t misread that. Hernandez shared the podium with ‘co-winners’

A lot happened at last weekend’s high school state track and field championship meet in
Clovis, Calif. Parents of cisgender student-athletes booed the one and only transgender
girl competing. Police and security officers showed up in large numbers to keep
protestors apart and safeguard the competitors. Police made an arrest outside the
stadium after a demonstrator brandishing a transgender pride flag allegedly assaulted a
man described as a conservative activist and caused damage to his vehicle.
The trans student — 16-year-old AB Hernandez — finished a winner. But she wasn’t “the” winner.
As CBS News reported, “Hernandez took home first place medals in both high jump and
triple jump and she placed second in the long jump event. Following a rule change by
the California Interscholastic Federation, a co-winner was named in each of the three
events in which Hernandez placed.”
You didn’t misread that. Hernandez shared the podium with “co-winners.”
As the Blade reported last week, the CIF introduced a new “pilot entry process” that for
the first time, allowed judges to score trans athletes separately from cisgender
competitors, so there were three winners in every event: a cisgender male winner, a
cisgender female winner and a trans student-athlete winner.
The new policy was announced hours after President Donald Trump threatened to pull
“large scale federal funding” from the state if officials allowed trans athletes to compete
according to their gender identity.
Despite the policy change, the U.S. Department of Justice announced on social
media it was investigating State Attorney General Rob Bonta, State Superintendent of
Public Instruction Tony Thurmond, the Jurupa Unified School District, and the CIF for
potential violations of Title IX, as the Blade reported.
So what happens now? As KXTV reported, President Trump issued another threat to
pull funding on Monday in a post to his Truth Social account, not naming Hernandez but
labeling her “a biological male” and using his favorite derogatory nickname for
California’s Democratic Gov. Gavin Newsom.
“A Biological Male competed in California Girls State Finals, WINNING BIG, despite the
fact that they were warned by me not to do so. As Governor Gavin Newscum fully
understands, large scale fines will be imposed!!!”
Now, the pundits are weighing-in. Sara Pequeño wrote in USA Today how she was
encouraged to see Hernandez share the 2nd place podium with Brooke White and “put
their arms around each other.”
“They’re setting an example for how all of us should treat our trans neighbors, i.e.,
treating them like human beings, not enemies,” she wrote.
As Pequeño noted, Save Women’s Sports, an anti-trans advocacy group, could only
identify five trans students in the entire United States who were competing on girls’
teams from kindergarten through grade 12 in 2023. “That group’s entire existence is to
hate trans athletes, and they found very little to hate,” she wrote.
According to the president of the NCAA, there are fewer than 10 student-athletes
who publicly identify as transgender out of the more than 500,000 competing at the
collegiate level.
Pequeño was not alone in finding joy in the rules change that brought cisgender and
transgender girls together on a podium, each of them a “co-winner.” So did self-
proclaimed “trans advocate” Cyd Zeigler.
He’s one of the co-founders of the LGBTQ+ sports site Outsports, who in 2023
infamously came close to endorsing Florida Republican Gov. Ron DeSantis for
president, only to offer his regrets, following a backlash from readers. Zeigler penned an
op-ed Wednesday originally titled “California trans athlete policy is something everyone
can embrace.”
“Everyone?” Not this sports editor.
He called the new CIF policy “the best possible path in 2025 to trans participation in
sports.”
In celebrating this change, Zeigler also trashed “goal-post-moving trans advocates” and
policies in California and Connecticut that allow “a trans girl to run in boys track meets
and, without a medical transition, later compete in girls meets,” meaning high school
competitions. “That’s bad policy,” declared Zeigler without evidence.
That policy in Connecticut has stood since 2011 and is enshrined in state law, and so far
has withstood legal challenges once again being heard in federal court.
Outsports at some point changed the headline of his screed to “New California trans
athlete policy is something we can embrace” and apparently made another significant
choice: Despite quoting the outlet’s one and only remaining transgender contributor,
Karleigh Webb, who opposes the rules change, Zeigler did not mention her by name.
Why?
In an article published before the championship, Webb wrote: “If AB Hernandez wins,
why should she have to share the spoils with someone else if’s not a tie? That’s what
professional transphobes like Jennifer Sey and Riley Gaines try to sell. Awarding a
duplicate medal gives their nonsense credence to the detriment of the sport and the
athletes.”
Webb is right. Zeigler and the CIF and Gov. Newsom are wrong. You either win, or you
lose, or if you prefer, you come in second, third, whatever. But “co-winners?”
That’s a crock.
Imagine if the Dodgers and Yankees shared the World Series trophy. Why shouldn’t the
49ers also win the Super Bowl alongside the Chiefs? Maybe Kamala Harris should be
declared a “co-winner” of last November’s election?
Personally, I’m glad to see Hernandez embraced by her cisgender peers. I’m relieved to
know that crowds cheering these amazing girls last weekend drowned out the hecklers
who showed up to boo a child. I’m encouraged that even if she had to share the win,
Hernandez was given her rightful place among the teens competing and proved she
was not only worthy of competing but did not win in every event.
So, she’s hardly “unbeatable.” Most trans athletes actually lose, as Zeigler wrote almost
six years ago, back before he started echoing anti-trans inclusion activists Martina Navratilova, Renee Richards and Nancy Hogshead-Makar.
If he really thinks the CIF “co-winners” rule is going to silence anti-trans forces, I think
he’s going to be very surprised by Riley Gaines and her crowd.
While it’s easy for Zeigler to concede public opinion has shifted, he should know
better than to blame those who pushed for inclusion, when it’s clear that conservative
voices in media and politicians, like his, are the ones responsible for influencing that
move to reject trans women’s right to compete in women’s sports. It’s a pendulum swing
that in time will undoubtedly swing back, once the science proves that trans women and
girls don’t always win. In fact, researchers have already proven some trans athletes are
at a disadvantage compared to their cisgender competitors.
Just as Parker Molloy reported that a Republican-commissioned study on gender
affirming care in Utah actually found “that youth who received care before age 18 had
better outcomes, especially around depression, anxiety and suicidality. Hormonal
treatments were associated with positive mental health and psychosocial functioning
outcomes.”
I believe the science is on the side of transgender Americans. Americans love a
winner. Not a “co-winner.”
Breaking News
Controversy brews in the City of Glendale over support of Pride event
Republican Mayor Ara Najarian pushes back on funding family-friendly Pride event

Over the last three weeks, glendaleOUT — a local LGBTQ group based in Glendale, California and city leadership have been at odds over securing financial support of a family-friendly Pride event set to happen on Saturday, June 7. As of Tuesday, Glendale’s city council voted 3-2 in favor of funding the event, ending a weeks-long argument over securing the funds.
The controversy began when the group highlighted how neighboring cities have visibly demonstrated support for Pride Month celebrations across the county, while the City of Glendale has yet to sponsor events with banners, city logos and financial sponsorship.
Councilmember Dan Brotman proposed $5,000 in sponsorship funds, noting that the city has funded other cultural events with much larger amounts.
Local leaders, but specifically Mayor Ara Najarian — who was just re-elected for a fifth term — are pushing back and opposing the proposal for funding. According to sources, Mayor Najarian openly opposed the proposal, stating a distant conflict of interest as the reason for the opposition.
LGBTQ advocates have been quoted as saying this is a “bad-faith political tactic, not grounded in any real conflict of interest.”
The next potential vote is expected to happen today at a city council meeting. Organizers say that the Pride event will happen regardless, but that they still hope to shed light on the patterns of sexual orientation-based discrimination in the city council.
For more information about the free community Pride event, visit glendaleOUT’s website.
-

 Theater3 days ago
Theater3 days agoGeffen Playhouse’s The Reservoir finds queer humor and joy in recovery and loss
-

 a&e features2 days ago
a&e features2 days agoHow this Texas drag king reclaimed their identity through Chicano-inspired drag
-

 Travel4 days ago
Travel4 days agoManchester is vibrant tapestry of culture, history, and Pride
-

 Obituary2 days ago
Obituary2 days agoThe queer community mourns the loss of trailblazer Jewel Thais-Williams
-

 a&e features3 days ago
a&e features3 days agoFrom Drag Race to Dvořák: Thorgy Thor takes the Hollywood Bowl for Classical Pride
-

 Books4 days ago
Books4 days agoEmbracing the chaos can be part of the fun
-

 Arts & Entertainment3 days ago
Arts & Entertainment3 days agoMary Lambert Returns With a Battle Cry in new single, “The Tempest”
-

 Television19 hours ago
Television19 hours agoICYMI: ‘Overcompensating’ a surprisingly sweet queer treat
-

 COMMENTARY2 days ago
COMMENTARY2 days agoUSAID’s demise: America’s global betrayal of trust






