Federal Government
Federal agency to investigate Trans student’s Title IX complaint
The Uni discriminated by directing her either to withdraw from classes or face discipline because she publicly identified as trans

PORTLAND, Or. – The Religious Exemption Accountability Project (REAP) received notice from the Office for Civil Rights (OCR) of the U.S. Department of Education (DOE) last week that the agency will initiate a Title IX investigation of Lincoln Christian University (LCU) based on a complaint REAP submitted to OCR last October on behalf of Kalie Hargrove, a trans woman and former LCU student.
OCR’s notification letter cited an allegation made in the complaint that “in August 2021, the University discriminated against Kalie Hargrove (Student A) on the basis of sex (gender identity) by directing her either to withdraw from classes or face discipline because she publicly identified as transgender.”
“We are glad to see the Office for Civil Rights take this important step in protecting the rights of LGBTQ+ students at taxpayer-funded religious colleges.” REAP founder and director, Paul Southwick said, adding: “However, we fear that the religious exemption to Title IX will likely result in the dismissal of Kalie’s complaint.”
Upon learning of the Title IX investigation, Hargrove commented, “You don’t want to get your hopes up because there’s no indication so far that the government gives a damn about LGBTQ+ students at religious schools.”
Hargrove has requested to join as a plaintiff in a class action lawsuit filed by the Portland, Oregon based REAP against the DOE last year challenging the constitutionality of the religious exemption to Title IX, a law prohibiting discrimination on the basis of sex by schools receiving Federal financial assistance.
Federal Government
USCIS announces it now only recognizes ‘two biological sexes’
Immigration agency announced it has implemented Trump executive order

U.S. Citizenship and Immigration Services on Wednesday announced it now only “recognizes two biological sexes, male and female.”
A press release notes this change to its policies is “consistent with” the “Defending Women from Gender Ideology Extremism and Restoring Biological Truth to the Federal Government” executive order that President Donald Trump signed shortly after he took office for the second time on Jan. 20.
“There are only two sexes — male and female,” said DHS spokesperson Tricia McLaughlin in a statement. “President Trump promised the American people a revolution of common sense, and that includes making sure that the policy of the U.S. government agrees with simple biological reality.”
“Proper management of our immigration system is a matter of national security, not a place to promote and coddle an ideology that permanently harms children and robs real women of their dignity, safety, and well-being,” she added.
The press release notes USCIS “considers a person’s sex as that which is generally evidenced on the birth certificate issued at or nearest to the time of birth.”
“If the birth certificate issued at or nearest to the time of birth indicates a sex other than male or female, USCIS will base the determination of sex on secondary evidence,” it reads.
The USCIS Policy Manuel defines “secondary evidence” as “evidence that may demonstrate a fact is more likely than not true, but the evidence does not derive from a primary, authoritative source.”
“Records maintained by religious or faith-based organizations showing that a person was divorced at a certain time are an example of secondary evidence of the divorce,” it says.
USCIS in its press release notes it “will not deny benefits solely because the benefit requestor did not properly indicate his or her sex.”
“This is a cruel and unnecessary policy that puts transgender, nonbinary, and intersex immigrants in danger,” said Immigration Equality Law and Policy Director Bridget Crawford on Wednesday. “The U.S. government is now forcing people to carry identity documents that do not reflect who they are, opening them up to increased discrimination, harassment, and violence. This policy does not just impact individuals — it affects their ability to travel, work, access healthcare, and live their lives authentically.”
“By denying trans people the right to self-select their gender, the government is making it harder for them to exist safely and with dignity,” added Crawford. “This is not about ‘common sense’—it is about erasing an entire community from the legal landscape. Transgender, nonbinary, and intersex people have always existed, and they deserve to have their identities fully recognized and respected. We will continue to fight for the rights of our clients and for the reversal of this discriminatory policy.”
Federal Government
Education Department moves to end support for trans students
Mental health services among programs that are in jeopardy

An email sent to employees at the U.S. Department of Education on Friday explains that “programs, contracts, policies, outward-facing media, regulations, and internal practices” will be reviewed and cut in cases where they “fail to affirm the reality of biological sex.”
The move, which is of a piece with President Donald Trump’s executive orders restricting transgender rights, jeopardizes the future of initiatives at the agency like mental health services and support for students experiencing homelessness.
Along with external-facing work at the agency, the directive targets employee programs such as those administered by LGBTQ+ resource groups, in keeping with the Trump-Vance administration’s rollback of diversity, equity, and inclusion within the federal government.
In recent weeks, federal agencies had begun changing their documents, policies, and websites for purposes of compliance with the new administration’s first executive action targeting the trans community, “Defending Women From Gender Ideology Extremism and Restoring Biological Truth to the Federal Government.”
For instance, the Education Department had removed a webpage offering tips for schools to better support homeless LGBTQ+ youth, noted ProPublica, which broke the news of the “sweeping” changes announced in the email to DOE staff.
According to the news service, the directive further explains the administration’s position that “The deliberate subjugation of women and girls by means of gender ideology — whether in intimate spaces, weaponized language, or American classrooms — negated the civil rights of biological females and fostered distrust of our federal institutions.”
A U.S. Senate committee hearing will be held Thursday for Linda McMahon, Trump’s nominee for education secretary, who has been criticized by LGBTQ+ advocacy groups. GLAAD, for instance, notes that she helped to launch and currently chairs the board of a conservative think tank that “has campaigned against policies that support transgender rights in education.”
NBC News reported on Tuesday that Trump planned to issue an executive order this week to abolish the Education Department altogether.
While the president and his conservative allies in and outside the administration have repeatedly expressed plans to disband the agency, doing so would require approval from Congress.
Federal Government
House races could decide Department of Education’s future
Second Trump administration could target transgender students

The Associated Press reports that more than a dozen races for seats in the U.S. House of Representatives, including 10 for congressional districts in California, remain too close to call as of Tuesday — a full week after voters cast their ballots on Nov. 5.
Democrats hope that if they can flip the lower chamber, which is now governed by a narrow Republican majority, it might function as a bulwark against President-elect Donald Trump, his incoming administration, and the 53-47 majority in the U.S. Senate that his party secured last week.
If, on the other hand, the GOP retains control of the House, the Republican victory would clear a major roadblock that could otherwise have stymied a major plank of Trump’s education agenda: Plans to permanently shutter the U.S. Department of Education.
Congress ultimately scuttled the former president’s effort to do so during his first administration — though, technically, the proposal then was to merge the agency with the U.S. Department of Labor.
The Wall Street Journal notes that some Republicans, at the time and in the years since, have come out against plans to abolish the 44-year-old agency, in some cases even objecting to major funding cuts proposed by Trump that they understood were likely be unpopular.
However, if the second term plans for DOE as delineated in the Trump campaign’s Agenda47 and the Heritage Foundation’s Project 2025 governing blueprint become a major policy priority once the incoming administration takes over in January, reluctant Republican lawmakers will face tremendous pressure to get out of Trump’s way.
Federal government will remain in schools to advance anti-trans, anti-woke agenda
Among other responsibilities, DOE disburses and manages student loans, enforces the civil rights laws in public schools, and provides funding for students with disabilities. The agency’s programs, such as Title I, offer assistance for low-achieving or high-poverty K-12 schools, while Pell Grants help undergraduates who otherwise would not be able to pay for college.
It is unclear whether or how those functions will continue if the DOE is disbanded.
Trump’s aim, at least in large part, is to give states — rather than the federal government — the ultimate say over how their schools are run. At the same time, perhaps paradoxically, the other cornerstone of his education policy agenda is to issue proscriptive rules governing the content, curricula, and classroom discussion that will be permitted in the country’s public schools.
Specifically, this means “critical race theory, gender ideology or other inappropriate racial, sexual or political” topics or materials are forbidden. Reasonable people are likely to disagree about what is and is not “inappropriate,” and they may well have different, even disparate, definitions for terms like “gender ideology.”
When Florida and other states enacted similar anti-LGBTQ content and curricular restrictions in their public schools, critics warned the ambiguous language in the statute and the resulting confusion would lead to censorship, or perhaps self-censorship, especially for students and staff who, by virtue of their skin color or sexual orientation or gender identity, are more likely to be targeted with targeted or overzealous enforcement in the first place.
DOE plays major role investigating alleged civil rights violations in schools
According to the National Education Association, “federal civil rights laws prohibit school boards and other employers from discriminating against or harassing staff or students based on their sexual orientation or gender identity,” which “means, for example, that a school district may not prohibit only LGBTQ+ educators from answering students’ questions about their families, may not prohibit recognition and discussion in class only of LGBTQ+ families, and may not require that only LGBTQ+ students hide their sexual orientation or gender identity at school.”
However, the NEA warns, “some school districts, administrators, and the Florida Department of Education may nonetheless choose to do so until a court orders otherwise.”
If officials at a public high school allow heterosexual teachers to display family photos in their classrooms but warn the openly gay teacher that he must put his away or be terminated for violating restrictions on in-school discussion of sexual orientation and gender identity, the manner in which the policy was enforced against him would presumably run afoul of the federal civil rights laws, which prohibit discrimination on the basis of sexual orientation.
The teacher could assume the expense of hiring an attorney to pursue legal remedies, shouldering the burden and the risk that litigation that could drag on for months and conclude with a judgment in favor of his employer. Alternatively, until or unless Trump dissolves the agency, he could file a complaint with DOE’s Office of Civil Rights.
Alternatively, until or unless Trump dissolves the agency, the teacher could file a complaint with DOE. The agency’s Office of Civil Rights would evaluate the information he shared to determine whether there were sufficient grounds to open an investigation and, if so, would deploy “a variety of fact-finding techniques” that can include a review of documentary evidence submitted by both parties, interviews with key witnesses, and site visits.
After the investigation is complete, if a “preponderance of the evidence supports a conclusion that the recipient failed to comply with the law,” OCR will attempt to negotiate a resolution agreement. If the recipient refuses to resolve the matter in this manner, OCR can “suspend, terminate, or refuse to grant or continue federal financial assistance to the recipient, or may refer the case to the Department of Justice.”
According to the DOE’s website, the agency has 11,782 investigations that were open as of Tuesday, with complaints against institutions of all kinds operating in all 50 states, from rural elementary schools in the Deep South to prestigious medical schools, community colleges, and charter schools for students with developmental disabilities. Likewise, the six civil rights laws over which OCR has jurisdiction cover a wide range of conduct, from sexual harassment to discrimination, retaliation, and single-sex athletics scholarships.
Should Trump succeed in abolishing the department, it is not yet clear how those active investigations will be handled, nor how complaints about violations of civil rights law by educational institutions would be reported and investigated moving forward in the agency’s absence.
During his first administration, Trump passed proposed changes to Title IX of the Education Amendments of 1972, which retooled the process for reporting sexual assault on college campuses in ways that were widely seen as imbalanced in favor of the accused.
President Joe Biden in April issued new guidelines that featured “significant shifts in how institutions address sexual harassment, and assault allegations while expanding protections for LGBTQ+ and pregnant students,” the American Council on Education wrote. Specifically, the administration provided a “new definition of sexual harassment, extending jurisdiction to off-campus, and international incidents,” while “clarifying protections against discrimination based on sexual orientation, gender identity, pregnancy, and parenting status.”
The regulations sidestepped thornier questions, however, about how schools should approach issues at the intersection of gender identity and competitive sports, specifying only that they should avoid bans that would categorically prohibit transgender athletes from participating.
Shortly after the Biden administration’s guidelines were introduced, Trump vowed they would be “terminated” on his first day in office. He also pledged to enact anti-trans policies that appear to have been modeled after some of the most extreme of the roughly 1,600 anti-trans bills that conservative statehouses have proposed from 2021-2024.
Among other promises Trump made during the campaign were plans to enact a nationwide ban on trans student athletes competing in accordance with their gender identity, a federal law that would recognize only two genders, and the prosecution of health care providers who administer gender affirming care to patients younger than 18.
Federal Government
Pentagon gives honorable discharges to 800+ LGBTQ+ veterans
Administration has committed to remedying harms of anti-LGBTQ military policies

Defense Secretary Lloyd Austin on Tuesday announced the Pentagon has upgraded the paperwork of more than 800 veterans who were discharged other than honorably before discriminatory policies like “Don’t Ask, Don’t Tell” were repealed.
“More than 96 percent of the individuals who were administratively separated under DADT and who served for long enough to receive a merit-based characterization of service now have an honorable characterization of service,” said Christa Specht, director of legal policy at the department’s Office of the Undersecretary of Defense for Personnel and Readiness.
The change will allow veterans to access benefits they had been denied, in areas from health care and college tuition assistance to VA loan programs and some jobs.
Separately, this summer President Joe Biden issued pardons to service members who had been convicted for sodomy before military laws criminalizing same-sex intimacy were lifted.
More than a decade after the repeal of “Don’t Ask, Don’t Tell,” the administration has made a priority of helping LGBTQ+ veterans who are eligible to upgrade their discharge papers, directing the department to help them overcome bureaucratic barriers and difficult-to-navigate processes.
However, as noted by CBS News, which documented the challenges faced by these former service members in a comprehensive investigation published last year, these efforts are ongoing.
The department is continuing to review cases beyond the 800+ included in Tuesday’s announcement, with an official telling CBS, “We encourage all veterans who believe they have suffered an error or injustice to request a correction to their military records.”
Federal Government
Administration officials visit LGBTQ-owned dental, medical offices
“There’s a surge in small businesses starting and that includes” those founded by members of the LGBTQ community”

WASHINGTON — The Assistant Secretary for Health Adm. Rachel Levine of the U.S. Department of Health and Human Services and Administrator Isabel Guzman of the U.S. Small Business Administration toured two LGBTQ-owned small businesses on Tuesday in Washington, D.C. — Big Gay Smiles and Price Medical, accompanied by the Washington Blade.
The event provided an “amazing opportunity” to “talk about the different synergies in terms of small businesses and the SBA, and health equity for many communities,” including the LGBTQ community, Levine told the Blade.
Representation matters, she said, adding, “that’s true in dental care and medical care,” where there is a tremendous need to push for improvements in health equity — which represents a major focus for HHS under her and Secretary Xavier Becerra’s leadership, and in the Biden-Harris administration across the board.
“Small businesses identify needs in communities,” Guzman said. With Big Gay Smiles, Dr. Robert McKernan and his husband Tyler Dougherty “have clearly identified a need” for “dentistry that is inclusive and that is respectful of the LGBTQIA community in particular.”
She added, “now that they’re a newly established business, part of the small business boom in the Biden-Harris administration, to see their growth and trajectory, it’s wonderful to know that there are going to be providers out there providing that missing support.”
The practice, founded in 2021, “is so affirming for the LGBTQIA community and we certainly wish them luck with their venture and they seem to have a great start,” Levine said. “They’re really dedicated to ending the HIV epidemic, providing excellent dental care, as well as oral cancer screenings, which are so important, and they’re really providing a real service to the community.”
Big Gay Smiles donates 10 percent of its revenue to national and local HIV/AIDS nonprofits. McKernan and Dougherty stressed that their business is committed to combatting homophobia and anti-LGBTQ attitudes and practices within the dental field more broadly.
“We try to align our practices here within this dental office to align with the strategic initiatives being able to help reduce HIV transmission, reduce stigma, and help to ensure people have the knowledge and [are] empowered to ensure that they’re safe,” Dougherty said.
McKernan added, “With the Academy of General Dentistry, we’ve done a lot of discussions around intersex, around trans affirming care, in order to help educate our fellow dental providers. It’s very important that every dentist here in the [D.C. area] provide trans affirming care and gender affirming care because it’s very important that someone who comes to a medical provider not be deadnamed, not get misnamed, and have an affirming environment.”
Trans and gender expansive communities face barriers to accessing care and are at higher risk for oral cancer, depression, and dental neglect. Levine, who is the country’s highest-ranking transgender government official, shared that she has encountered discrimination in dental offices.
After touring the office, Levine and McKernan discussed the persistence of discrimination against patients living with HIV/AIDS by dental practices, despite the fact that this conduct is illegal.
“I’ve traveled around the country,” the assistant health secretary told the Blade. “We have seen that many FQHCs [federally qualified health centers] or community health centers as well as LGBTQIA community health centers have had dentists, like Whitman-Walker, to provide that care because many people with HIV and in our broader community have faced stigma and have not been able to access very, very important dental care.”
Prior to opening his practice, McKernan practiced dentistry at Whitman-Walker, the D.C. nonprofit community health center that has expertise in treating LGBTQ patients and those living with HIV/AIDS. Big Gay Smiles is a red ribbon sponsor for the organization’s Walk & 5K to End HIV.
After their visit with Big Gay Smiles, Levine and Guzman headed to Price Medical, a practice whose focus areas include internal medicine/primary care, HIV specialty care, immunizations, infectious disease treatment, and aesthetics like Botox.
There, the officials talked with Dr. Timothy Price about his office’s work advancing health equity and serving LGBTQ patients including those living with HIV/AIDS, as well as the ways in which small businesses like his have benefitted from access to electronic health records and telemedicine.

(Washington Blade photo by Christopher Kane)
“People being able to access medical care from the comfort of their home or workplace can be very important,” Price said, with technology providing the means by which they can “ask questions and get an answer and have access to a health care provider.”
Often, LGBTQ patients will have concerns, including sexual health concerns, that need urgent attention, he said. For instance, “we’ve had patients need to access us for post-exposure prophylaxis for HIV,” in some cases when “people are vacationing and they have something that might be related to their health and they can reach us [via telemedicine] so that’s the way it’s really helped us and helped the patients.”
Access to technology for small businesses is an area in which the SBA can play a valuable role, Guzman noted.
“The Biden-Harris administration has focused on a whole-of-government approach to making sure we can support the community, and that includes in entrepreneurship,” she told the Blade.
“There’s a surge in [small] businesses starting and that includes” those founded by members of the LGBTQ community “and so you see that there’s products and services that need to be offered,” and the administration is “committed to making sure that we can fund those great ideas.”
Guzman said she sees opportunities for future collaboration between her agency and HHS to help encourage and facilitate innovation in the healthcare space. “Small businesses are innovators creating the future of health tech,” she said.
Levine agreed, noting “we have been talking about that, about different ways that we can work together, because as we think about the social determinants of health and those other social factors that impact health, well, economic opportunity is absolutely a social determinant of health,” and small businesses are certainly a critical way to broaden economic opportunity.
Federal Government
HHS plans to expand health equity in second Biden-Harris admin
Secretary Xavier Becerra and Assistant Health Secretary Adm. Rachel Levine detailed plans to expand health equity initiatives

WASHINGTON — Speaking with the Washington Blade on Monday, U.S. Department of Health and Human Services Secretary Xavier Becerra and Assistant Health Secretary Adm. Rachel Levine detailed plans to expand health equity initiatives under a second Biden-Harris administration.
The conversation came shortly after the agency held a Progress Pride flag-raising ceremony, where U.S. Rep. Mark Takano (D-Calif.), delivered opening remarks alongside the top HHS officials who also spoke at the department’s second annual Pride Summit later on Monday.
Levine highlighted a slate of recent actions and goals on which to build in a second term: The issuance in April of a final rule clarifying that discrimination on the basis of sexual orientation and gender identity is prohibited under the Affordable Care Act; a demographic data collection plan on sexual orientation and gender identity metrics; the pursuit of regulations and litigation (coordinated with the Justice Department) to combat healthcare restrictions, including those which target LGBTQ communities; and the agency’s commitments to diversity, equity, inclusion, and accessibility.
“To put an exclamation point behind some of that,” Becerra said, “on SOGI, we think it’s important to gather the data that lets us figure out where to go next, or where you have issues” in “getting access to the care that you need.”
“And we know we’re going to end up in court with a lot of the rules that we’ve enacted,” added the secretary, who previously served as attorney general of California, “but we’re ready for that — they’ll get tested, and we’re ready to defend [them].”
Becerra added that along with the initiatives outlined by Levine, HHS is looking to expand efforts in the behavioral health space to maximize opportunities to match patients with providers who have shared backgrounds, identities, and lived experiences.
That way, he said, “chances are that individual in need of care is going to open up faster. So we’re going to try to move quicker towards providing, in the behavioral health setting, people with lived experiences who can speak to what this individual is hurting from, is suffering from, so we can try to help them with their behavioral health challenges.”
The secretary praised the Biden-Harris administration’s pro-equality record, noting, “the fact that we’re the first department to fly the Pride flag, I think it shows that we’re out front, and we are very intent on making sure everyone has access to the care that they need.”
“And to do that, you’re going to find yourself in court,” Becerra said. “To do that, you need to do an aggressive job of collecting data. To do that, you have to show people that you can approach them with someone who’s experienced in what they’re going through. And so all of those things have to be amped up if we’re going to make further progress in the next administration’s four years.”
Levine repeatedly credited the secretary’s leadership as well as President Joe Biden’s work advancing equity throughout his administration, including through executive orders, when discussing HHS’s efforts to expand healthcare access and improve health outcomes for diverse populations including the LGBTQ community.
“One of the highlights, I think, of the Biden-Harris administration and Secretary Becerra’s leadership is the the emphasis on building representation in Washington that looks like the people of our country,” said Levine, who became the highest-ranking transgender government official with her appointment as assistant health secretary in 2021.
“Whether it is communities of color, whether it is the LGBTQI+ community, young people, seniors, I mean, we really want the the people who work for the people of our country to look like them and to represent them,” she said.
She also highlighted the extent to which her and Becerra’s work on this front has involved putting boots on the ground. “I’ve been to Austin. I’ve been to Dallas. I’ve been to Nashville. I’m going to Jacksonville. We tried to get to Idaho to Boise, but we got snowed out.”
“We are everywhere,” Levine said, adding that she likes to say the secretary has been doing “everything, everywhere, all at once,” (the title of a critically acclaimed film that won the Academy Award for Best Picture in 2023.)
In a Pride month press release shared by the agency on Monday evening, Becerra said in a statement, “HHS works every day to build an America where LGBTQI+ Americans have access to quality, affordable health care and can go to the doctor without fear of stigma or discrimination. Where the state you live in doesn’t determine whether you can access lifesaving, gender-affirming care. And where more communities embrace the diversity that has always strengthened our national character.”
“Pride reminds us that we are a strong, resilient, and powerful community that fights hate with love,” Levine said. “As we celebrate Pride Month, we should recognize how far we have come, even as we take stock of the challenges that we face. Everything we do at HHS emphasizes health equity and this pride month, we are making a focused effort to address and eliminate the health disparities within the LGBTQI+ community.”
She added, “We are focused on our efforts to end the HIV epidemic in the U.S., prevent syphilis and congenital syphilis, and promote access to care for LGBTQI+ people across America. Together, we can work to support healthy people, healthy communities, and a healthy nation for all. I am a positive and optimistic person, and I believe that working together, we can create a healthier, better future for all people living in the United States.”
Federal Government
Federal departments & agencies celebrate LGBTQ+ Pride Month
Federal Agencies mark Pride: “To the LGBTQI+ community: We see you, we stand with you, and we celebrate you with pride”

WASHINGTON – As Pride Month officially kicks off with the proclamation issued by President Joe Biden on May 31, in addition to the new White House LGBTQ+ Community Safety Partnership launching a new guide containing key federal resources, the Executive Branch’s heads and agencies also are honoring Pride.
From social media:
This #PrideMonth, the Department of Justice celebrates all members of Lesbian, Gay, Bisexual, Transgender, Queer, and Intersex community.
— U.S. Department of Justice (@TheJusticeDept) June 1, 2024
Read more: https://t.co/KYMZY2pt3m pic.twitter.com/cnm0KOXhr1
Pride Month is a time to come together to honor the contributions of LGBTQ+ service members. We are committed to ensuring and promoting an atmosphere of dignity and respect for all civilian and military personnel. pic.twitter.com/RyGTDBjUcN
— Department of Defense 🇺🇸 (@DeptofDefense) June 1, 2024
Despite great strides for equality around the world, LGBTQI+ persons in many countries continue to face grave danger. Protecting the spirit of #Pride and promoting respect for the human rights of LGBTQI+ persons is central to the work we do. pic.twitter.com/vN4Sz19a2Y
— Department of State (@StateDept) June 1, 2024
Happy Pride! Pride month rests on a tradition of both celebration and protest, and above all an insistence on equality, safety, and belonging for all LGBTQ+ Americans.
— Secretary Pete Buttigieg (@SecretaryPete) June 1, 2024
June marks Pride Month, a time to celebrate the many contributions of our LGBTQIA+ workforce and stand with the community – our community – throughout the month and every day. #WeAreDHS #Pride2024 pic.twitter.com/rVwhFXnAT1
— Homeland Security (@DHSgov) June 3, 2024
This Pride Month and every month, the @Interior team works to build a country that represents and welcomes each of us. I'm honored to celebrate our vibrant LGBTQI+ community worldwide and their countless contributions to our shared, equitable future. Happy Pride Month, everyone! pic.twitter.com/6OU55MoQkE
— Secretary Deb Haaland (@SecDebHaaland) May 31, 2024
Let us Reflect, Empower and Unite together this #Pride Month as we celebrate the diversity of the NWS family! Their skills and perspectives allow us to meet our mission of protecting a diverse nation. pic.twitter.com/Z5nnweZRFX
— National Weather Service (@NWS) June 1, 2024
Happy #PrideMonth 🏳️🌈! This annual occasion is a celebration of the strength, vibrancy, and diversity of the #LGBTQI+ community. Read a message from ITA Office of Public Affairs Director Tyrik McKeiver on the importance of building an inclusive world ➡️ https://t.co/vubtGYYKmS
— ITA (@TradeGov) June 3, 2024
It's #PrideMonth!
— U.S. Commerce Dept. (@CommerceGov) June 3, 2024
Tune in as we share stories of our @CommerceGov colleagues and resources available to the LGBTQI+ community. pic.twitter.com/pMJUE9r3P5
June is LGBTQ+ Pride Month!
— U.S. Coast Guard (@USCG) June 1, 2024
It’s a reflection on LGBTQ+ history, resilience, and progress. This month, and every month, the USCG stands in solidarity for the inclusivity and acceptance of all our shipmates!#EqualityForAll #equality #Pride #pridemonth pic.twitter.com/D3JQsnDxxN
💙🇺🇸 Charting a course for equality. Celebrate with the #USNavy this #PrideMonth! ⚓ pic.twitter.com/B77qWaXBd9
— U.S. Navy (@USNavy) June 3, 2024
This #PrideMonth, we honor the contributions of LGBTQ+ personnel across the Joint Force. Their service enriches our military community and ensures we are ready to respond when the Nation calls. pic.twitter.com/roejPri7qb
— The Joint Staff 🇺🇸 (@thejointstaff) June 3, 2024
During #PrideMonth, we celebrate the courage and resilience of the LGBTQI+ community and stand beside them in the fight against hatred and bigotry. Today and every day, HHS remains deeply committed to protecting the rights of LGBTQI+ Americans. pic.twitter.com/yFqpiYjSiu
— HHS.gov (@HHSGov) June 1, 2024
Today, we kicked off our Second Annual HHS Pride Summit!
— HHS.gov (@HHSGov) June 3, 2024
At HHS, we are committed to delivering on the promise of health equity for communities that have often been underserved and under-resourced, including the LGBTQI+ community. #PrideMonth pic.twitter.com/fhD1p4Ce70
At @USDOL, we're committed to promoting opportunity for all workers. 🌈 Proudly standing with LGBTQI+ workers, we recognize their invaluable contributions to diverse and inclusive workplaces. #PrideMonth #Equality pic.twitter.com/4ubvUO3BMk
— U.S. Department of Labor (@USDOL) June 1, 2024
This Pride Month—and every day—VA openly and proudly recognizes the more than one million LGBTQ+ Veterans that have served this nation. We thank each and every one of them—and every person who has donned the uniform—for their service and sacrifice. pic.twitter.com/3gIOWgIYLe
— Veterans Affairs (@DeptVetAffairs) June 1, 2024
As we mark the start of #PrideMonth, we're reflecting on how far we've come while recognizing the work still to be done.
— NASA (@NASA) June 1, 2024
There's space for everyone — all genders and orientations — in exploration and discovery. pic.twitter.com/8k7Hrcy5uz
Every student should feel safe attending school in America, free from discrimination & valued for who they are.
— U.S. Department of Education (@usedgov) June 1, 2024
That means creating welcoming & safe learning environments for every LGTBQI+ student & ensuring educators have resources to support LGBTQI+ young people.#PrideMonth pic.twitter.com/bIXWIFTjyn
To the LGBTQI+ community: We see you, we stand with you, and we celebrate you with pride. pic.twitter.com/Aj813ss9os
— Vice President Kamala Harris (@VP) June 2, 2024
This Pride Month, we thank LGBTQ+ Service members for their contributions to our national security. As Secretary of Defense, I remain dedicated to making sure that our LGBTQ personnel across the Joint Force can continue to serve with dignity and respect. We applaud your service –… pic.twitter.com/hs61oK6lzR
— Secretary of Defense Lloyd J. Austin III (@SecDef) June 3, 2024
This month, we uplift the achievements and resiliency of the LGBTQI+ community. USDA is committed to continue fostering cultural competency within our workforce and advancing equality for all we serve.
— Dept. of Agriculture (@USDA) June 1, 2024
Happy Pride Month! pic.twitter.com/YVEMQqO9ax
At the #FBI, we know that diversity makes us stronger. During #PrideMonth, the FBI celebrates our #LGBTQIA+ colleagues’ contributions to our country and our mission. Learn more about the Bureau’s diversity and inclusion initiatives: https://t.co/qYeLcYObNb #PRIDE pic.twitter.com/c7jqFrvMYp
— FBI (@FBI) June 3, 2024
Happy Pride Month!
— The White House (@WhiteHouse) June 1, 2024
This month and every month, our Administration celebrates the extraordinary courage of LGBTQI+ people and proudly stands with them in the fight for equality, justice, and inclusion. pic.twitter.com/bgHLLHNjS1
June is LGBTQ+ Pride Month, and all month long, we’ll be sharing photos and a little bit about our National Guard members to celebrate our diverse force. Learn more: https://t.co/jOSfDDKhyV #PrideMonth pic.twitter.com/aAZ5NYT0pW
— National Guard (@USNationalGuard) June 1, 2024
The aviation industry thrives and maintains safety through the diversity of its participants, including the LGBTQIA+ community. #PrideMonth pic.twitter.com/eSd5xfO1e0
— The FAA ✈️ (@FAANews) June 1, 2024
We raise the pride flag at @usedgov to celebrate Pride Month and the strength of the LGBTQI+ community. But it also serves as a reminder that in a world where too many LGBTQI+ students are still bullied for who they are, we’ve got work to do. Hate has no place in our classrooms. pic.twitter.com/MPIqJuupqv
— Secretary Miguel Cardona (@SecCardona) June 6, 2024
Federal Government
Justice Dept. investigating anti-trans violence at Virginia school
Norfolk Public Schools Superintendent Sharon Byrdsong declined an interview request. The U.S. Attorney’s Office did not comment

WASHINGTON – The U.S. Justice Department has reportedly launched an investigation into violence against transgender and Latino students in Norfolk, Va.
WHRO, a public radio broadcast radio station, reported Melissa Corrigan earlier this year spoke with an attorney from the Justice Department’s Civil Rights Division about violence that her trans son experienced at Norview High School. The Hampton Roads public radio station said Corrigan contacted the U.S. Attorney’s Office for the Eastern District of Virginia in Norfolk more than a year ago.
Corrigan told WHRO that her son suffered harassment, physical violence because of his gender identity. She also said he was sexually assaulted in a bathroom.
“He was definitely feeling targeted because of it,” Corrigan told WHRO, referring to her son’s gender identity. “And more than that, he wasn’t feeling like he was getting any protection from administration.”
Corrigan said her son eventually withdrew from Norfolk Public Schools. She said a Justice Department Civil Rights Division attorney met with her and her son for two hours in March.
WHRO also reported Latino students at Norview High School said they had been assaulted because of their race. Their families, like Corrigan, said administrations did nothing to stop the violence.
The Biden-Harris administration has said Title IX of the Education Amendments of 1972 prohibits discrimination in schools based on gender identity and sexual orientation. Republican Virginia Attorney General Jason Miyares is among the state attorneys general who have challenged new Title IX rules that expand protections for LGBTQ students.
WHRO reported Norfolk Public Schools Superintendent Sharon Byrdsong declined an interview request. The local U.S. Attorney’s Office did not confirm whether an investigation is underway.
Federal Government
National Park Service clarifies uniform policies for all events
National Park Service issued a memo clarifying uniform policies for employees from attending any event.

By Erin Reed | WASHINGTON – The National Park Service on May 17 clarified its policy on employees wearing official uniforms to non-sanctioned events, which has implications for Pride events.
It’s unclear what triggered the clarification. A source at the National Park Service told the Blade in a statement that the uniform policy “has not changed,” but some LGBTQ employees report feeling betrayed and note that official Pride participation in major cities is uncertain as applications to participate in parades remain unprocessed.
The clarification comes amid increasing crackdowns on Pride flags and LGBTQ people nationwide.
The announcement was first disclosed in a memo to park service employees that did not directly address Pride but stated that “requests from employees asking to participate in uniform in a variety of events and activities, including events not organized by the NPS” conflict with park service policy.
The specific provision cited states that park service employees cannot wear the uniform to events that would construe support for “a particular issue, position, or political party.” Applying this provision to bar Pride participation drew ire from some LGBTQ employees who assert that LGBTQ Pride is not about an “issue, position, or political party,” but about identity and diversity. The employees, who spoke on condition of anonymity, also pointed out that the internal ERG guide allowed for participation in Pride events and that park employees had participated in Pride events with approval for years under the current set of rules.

In a follow-up, the park service stated that the ERG resource known as the “OUTsiders Guide to Pride” conflicts with its policy and that it is in discussion with ERG leaders to review it and similar documents.
Meanwhile, it stated that park service participation in Pride “could imply agency support … on a particular issue of public concern,” essentially stating that celebrations of LGBTQ employees would be considered an “issue of public concern” rather than a non-political celebration of diversity. As such, they determined that park service official participation in parades “should be extremely limited.”
Concern spread among some park service employees . They noted that the park service has participated in Pride parades across the United States for years under the same set of rules, including during the Trump administration, which notably cracked down on LGBTQ Pride in government agencies, such as at embassies abroad.
They also noted that Stonewall National Monument is run by the park service. Importantly, Stonewall National Monument’s founding documents state, “The purpose of Stonewall National Monument is to preserve and protect Christopher Park and the historic resources associated with it and to interpret the Stonewall National Historic Landmark’s resources and values related to the lesbian, gay, bisexual, and transgender civil rights movement.”
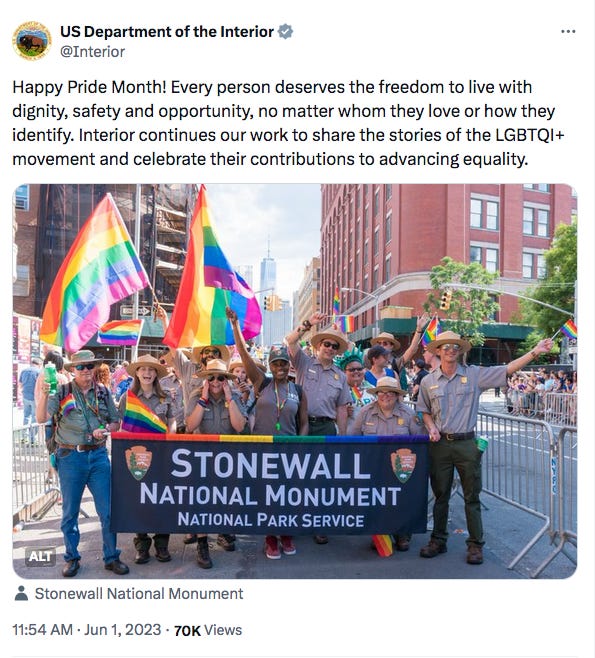
One park service employee, speaking on condition of anonymity, stated that multiple Pride parade requests are currently sitting on desks “collecting dust” for participation and representation in major city Pride festivities. When asked about the determination that Pride festivals are an “issue of public concern,” they said, “Pride is not political, it’s not a cause, you just are LGBTQ+. It’s a celebration of who we are.” They added, “Morale is just so low right now. There’s not a lot of fight left in us.”
The Blade reached out to a park service spokesperson to ask about Pride parades in major cities and whether the park service would continue participating this year as they have in previous years. The spokesperson stated that the policy “had not changed” and that “Previous interpretations of the uniform policy were inconsistent and, as you can imagine, approving participation in some events and not others could be seen as discrimination based on viewpoint.” They added that in-park Pride events have not been canceled and that community events outside of the parks that “directly relate to a park’s mission” could be approved. However, they did not indicate whether these events would include continued contingents in major U.S. city Pride parades and celebrations and could not be reached for a follow-up on this question.
Park service resources currently live on the site call for people to “Celebrate Pride,” citing Stonewall National Monument to state that “The LGBTQ experience is a vital facet of America’s rich and diverse past.” This resource emphasizes the importance of not rendering LGBTQ people invisible, stating, “By recovering the voices that have been erased and marginalized, the NPS embarks on an important project to capture and celebrate our multi-vocal past.”
Park Service employees have marched in uniform for years. According to the Bay Area Reporter, in 2014, Christine Lenhertz of the park service requested that a group of LGBTQ park service employees be allowed to wear their uniforms in the Pride parade. They were initially barred from doing so, prompting the group to file a complaint. She then sought a ruling from the Office of the Solicitor for the Department of the Interior, who ruled that there was no reason to bar her and other LGBTQ people from participating in uniform. Since then, many park service contingents have participated in Pride events.

The future of Pride parade participation with in-uniform park service employees is uncertain. While it appears that there will be some Pride events in certain national parks, such as Stonewall, external participation in major city Pride events seems to be on hold in at least some major American cities.
You can see the full response to the request for comment from a park service spokesperson here:
The NPS uniform policy has not changed. There are no restrictions on wearing of uniforms in NPS-organized in-park events. There has been no directive to cancel NPS-organized in-park events. Superintendents have discretion to approve park-organized events, which support park purpose and mission, and departmental mission, initiatives, and priorities (e.g., diversity, inclusion, climate change, and tribal engagement.) This would include many of the events planned to celebrate Pride month.
Official NPS participation in community events that directly relate to a park’s mission can be approved by the park superintendent, provided it is consistent with applicable laws, rules, regulations, and NPS policies.
Last week, the service sent out a reminder about the uniform policy — specifically because there has been an in-flux of requests from folks asking to wear their uniforms for non-park service events. These requests run the gamut of topics, but could include weekend, off duty events that folks are of course able to do in their personal capacity, but not while wearing a uniform representing the federal government. Previous interpretations of the uniform policy were inconsistent and as you can imagine, approving participation in some events and not others could be seen as discrimination based on viewpoint.
NPS employees represent a diversity of identities, cultures, and experiences, and we are committed to supporting all of our workforce. Like any large organization, we have a diverse workforce supporting myriad causes, and we welcome employees to express their personal support for various issues, positions, and political parties, provided they do not imply their presence or endorsement constitutes official NPS support for the same. And, also like other large organizations, there are limits to what employees can do while on-duty and in uniform and seen as communicating on behalf of the NPS.
******************************************************************************************

Erin Reed is a transgender woman (she/her pronouns) and researcher who tracks anti-LGBTQ+ legislation around the world and helps people become better advocates for their queer family, friends, colleagues, and community. Reed also is a social media consultant and public speaker.
******************************************************************************************
The preceding article was first published at Erin In The Morning and is republished with permission.
Federal Government
CDC issues warning on new “deadlier strain” of Mpox
As LGBTQ+ Pride month and events happen globally, there is more need for greater caution and people to take steps at prevention
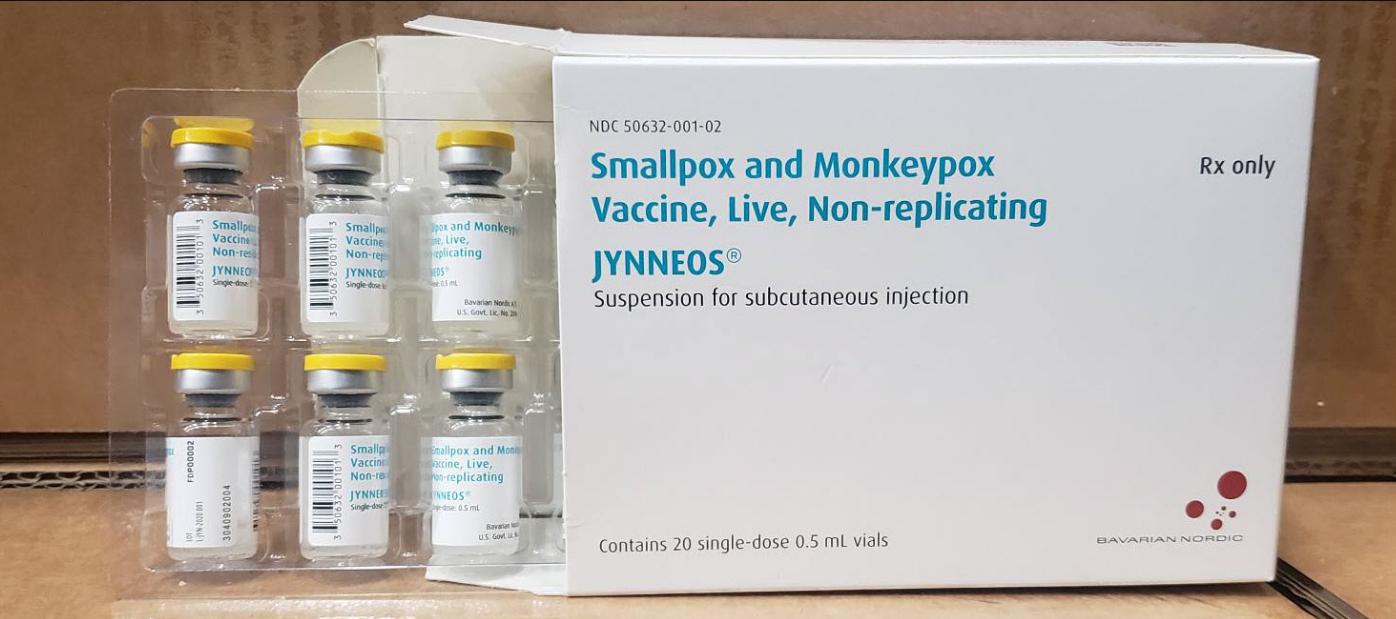
ATLANTA, Ga. – The Centers for Disease Control and Prevention (CDC) have issued a health advisory regarding a deadlier strain of the Mpox virus outbreak which is currently impacting the Democratic Republic of Congo (DRC).
According to the CDC, since January of 2023, DRC has reported more than 19,000 suspect mpox cases and more than 900 deaths. The CDC stated that the overall risk to the United States posed by the clade I mpox outbreak is low.
The risk to gay, bisexual, and other men who have sex with men (MSM) who have more than one sexual partner and people who have sex with MSM, regardless of gender, is assessed as low to moderate the agency stated.
While no cases of that subtype have been identified outside sub-Saharan Africa so far, the World Health Organization said earlier this week that the escalating epidemic in Congo nevertheless poses a global threat, just as infections in Nigeria set off the 2022 outbreak according to a WHO spokesperson.
The spokesperson also noted that as LGBTQ+ Pride month and events happen globally, there is more need for greater caution and people to take steps at prevention including being vaccinated.
The CDC advises that while there are no changes to the overall risk assessment, people in the United States who have already had Mpox or are fully vaccinated should be protected against the type of Mpox spreading in DRC. Casual contact, such as might occur during travel, is not likely to cause the disease to spread. The best protection against Mpox is two doses of the JYNNEOS vaccine.
The CDC also noted the risk might change as more information becomes available, or if cases appear outside DRC or other African countries where clade I exists naturally.
-

 Theater4 days ago
Theater4 days agoGeffen Playhouse’s The Reservoir finds queer humor and joy in recovery and loss
-

 COMMENTARY3 days ago
COMMENTARY3 days agoUSAID’s demise: America’s global betrayal of trust
-

 a&e features3 days ago
a&e features3 days agoHow this Texas drag king reclaimed their identity through Chicano-inspired drag
-

 Obituary3 days ago
Obituary3 days agoThe queer community mourns the loss of trailblazer Jewel Thais-Williams
-

 Television2 days ago
Television2 days agoICYMI: ‘Overcompensating’ a surprisingly sweet queer treat
-

 a&e features4 days ago
a&e features4 days agoFrom Drag Race to Dvořák: Thorgy Thor takes the Hollywood Bowl for Classical Pride
-

 Arts & Entertainment4 days ago
Arts & Entertainment4 days agoMary Lambert Returns With a Battle Cry in new single, “The Tempest”
-

 Opinions18 hours ago
Opinions18 hours agoThe psychology of a queer Trump supporter: Navigating identity, ideology, and internal conflict
-

 Living16 hours ago
Living16 hours agoFaithfully queer: Finding God and growth in Modern Orthodoxy
-

 Breaking News3 hours ago
Breaking News3 hours agoTrump administration sues California over trans student-athletes





